Mini strokes, also known as transient ischemic attacks (TIAs), are brief episodes of neurological dysfunction caused by a temporary interruption of blood flow to the brain. While they may not cause permanent damage themselves, TIAs are often warning signs of a potential future stroke. Therefore, understanding the process of mini stroke recovery is crucial for individuals who have experienced such an event. In recent years, biofeedback has emerged as a promising therapeutic approach in stroke rehabilitation, including for TIA mini stroke recovery at home. This article explores the concept of mini stroke recovery and the role of biofeedback in aiding the rehabilitation process.
What is a Mini Stroke?
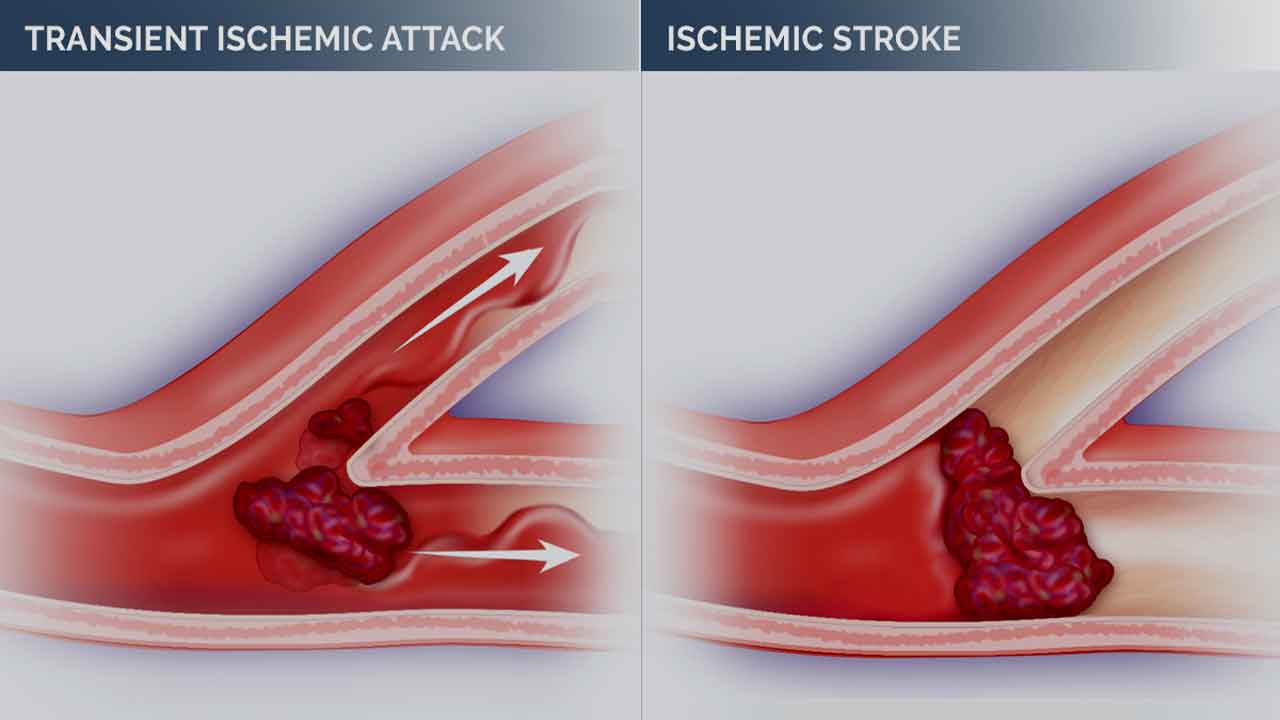
A mini-stroke, clinically referred to as a transient ischemic attack (TIA), is a temporary disruption of blood flow to a part of the brain. Despite its transient nature, a mini-stroke produces symptoms similar to a full stroke, albeit typically lasting for a shorter duration. These symptoms arise from sudden deprivation of oxygen and nutrients to brain cells, leading to temporary dysfunction.
Symptoms of a mini-stroke often involve sudden weakness or numbness in the face, arm, or leg, typically affecting one side of the body. Additionally, individuals may experience difficulty speaking or understanding speech. There may also be temporary loss of vision in one or both eyes. Dizziness, along with loss of balance or coordination, can occur as well.
Unlike a full stroke, the symptoms of a mini-stroke (TIA) usually resolve within minutes to hours and leave no permanent damage. However, TIAs are often considered warning signs of a potential future stroke and should be taken seriously. It’s crucial to seek medical attention promptly if you suspect you or someone else is experiencing a mini-stroke.
Causes and Risk Factors of Mini Stroke
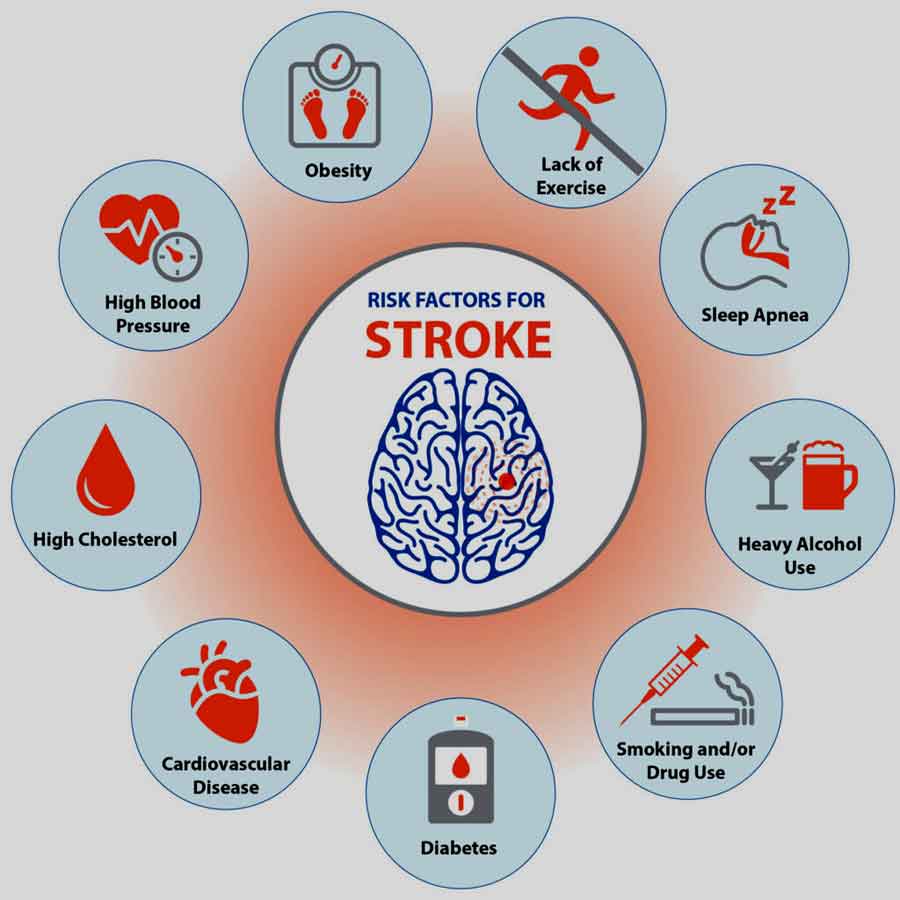
Mini strokes occur when blood vessels supplying the brain temporarily block or narrow. Common causes include blood clots, atherosclerosis (hardening and narrowing of arteries), or embolisms (traveling blood clots). Risk factors for mini-strokes mirror those for full strokes and include hypertension, diabetes, smoking, high cholesterol, obesity, and a sedentary lifestyle.
Understanding the causes and risk factors associated with mini-strokes, or transient ischemic attacks (TIAs), is essential for identifying individuals at higher risk and implementing preventive measures.
Atherosclerosis:
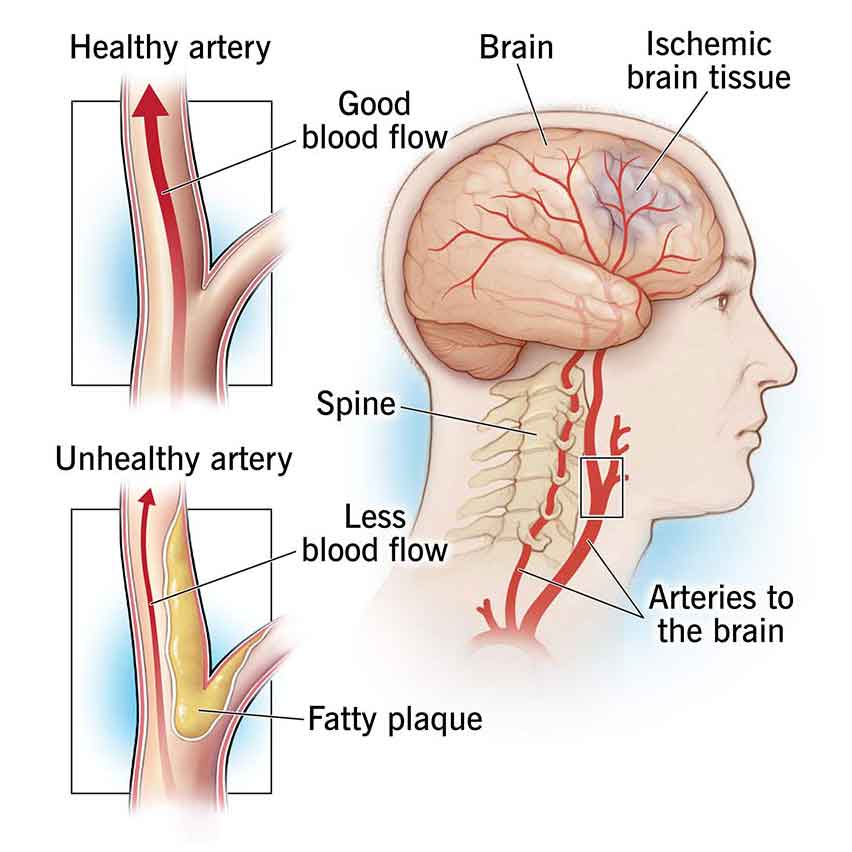
Atherosclerosis refers to the buildup of fatty deposits (plaques) in the arteries, leading to narrowing and hardening of the blood vessels. These plaques can reduce blood flow to the brain, increasing the risk of mini-strokes. Risk factors for atherosclerosis include high cholesterol, high blood pressure, smoking, diabetes, and obesity.
Blood Clots:
Blood clots, also called thrombi, can develop within blood vessels that supply the brain or other body parts. These clots may travel to the brain and cause a blockage. Certain conditions increase the risk of blood clot formation, such as atrial fibrillation and an irregular heart rhythm. Heart valve disorders and deep vein thrombosis (DVT) also heighten this risk. Additionally, specific medical procedures or conditions like surgery, cancer, or extended periods of immobilization can further raise the chances of experiencing mini-strokes.
Embolism:
An embolism happens when a blood clot or debris detaches from its original site and moves through the bloodstream. It travels until it gets stuck in a smaller blood vessel, where it blocks blood flow. Cardiac sources of emboli include atrial fibrillation, heart valve disorders (such as mitral valve stenosis), and recent heart attacks. Non-cardiac sources may consist of blood clots originating from the carotid arteries in the neck or other peripheral arteries.
Hypertension (High Blood Pressure):
Chronic high blood pressure can damage the blood vessel walls over time, increasing the risk of atherosclerosis and blood clot formation. Uncontrolled hypertension is a significant risk factor for both ischemic and hemorrhagic strokes, including mini-strokes.
Diabetes Mellitus:
Diabetes is associated with various vascular complications, including damage to blood vessels and increased clotting tendencies. Individuals with diabetes have a higher risk of developing atherosclerosis and hypertension, further predisposing them to mini-strokes.
Smoking:
Smoking cigarettes significantly increases the risk of cardiovascular disease, including atherosclerosis and blood clot formation. The chemicals in tobacco smoke damage blood vessel walls, promote inflammation, and contribute to plaque buildup.
High Cholesterol:
Elevated LDL (low-density lipoprotein) cholesterol levels, often called “bad” cholesterol, contribute to the formation of atherosclerotic plaques. These plaques can narrow the arteries and impede blood flow to the brain, increasing the risk of mini-strokes.
Age and Gender:
The risk of mini-strokes increases with age, making older adults more susceptible. Men are at a slightly higher risk of experiencing mini-strokes than women, although the risk for women increases after menopause.
Family History and Genetics:
A family history of stroke or cardiovascular disease can increase an individual’s predisposition to mini-strokes. Certain genetic factors may also influence an individual’s susceptibility to developing vascular conditions, predisposing them to mini-strokes.
Lifestyle Factors:
A sedentary lifestyle, poor diet, excessive alcohol consumption, and stress can contribute to the development of risk factors such as obesity, hypertension, and high cholesterol, thereby increasing the risk of mini-strokes.
Recognizing these causes and risk factors is crucial for implementing preventive measures and lifestyle modifications to reduce the likelihood of experiencing a mini-stroke. Additionally, managing underlying medical conditions and adopting a healthy lifestyle can help mitigate the risk of future vascular events.
Pathophysiology of Mini Stroke
A mini-stroke, or transient ischemic attack (TIA), occurs through similar pathophysiological mechanisms as a full stroke (cerebrovascular accident or CVA). However, the critical difference is that TIA symptoms are temporary and typically resolve within 24 hours. Understanding the pathophysiology of a mini-stroke involves examining the underlying causes and mechanisms that lead to transient neurological symptoms.
Ischemic Pathophysiology:
Most mini-strokes are ischemic due to a temporary interruption of blood flow to a part of the brain. A temporary blockage or narrowing of a cerebral artery often causes this interruption. Common causes of ischemic mini-strokes include emboli, clots, or debris that travel to the brain from other parts of the body, like the heart or carotid arteries. Another cause is local thrombosis, which involves the formation of a blood clot within a cerebral artery. Both mechanisms can disrupt blood flow to the brain, triggering a mini-stroke.
Embolic Mini Strokes:
Embolic mini-strokes often occur when a clot or debris dislodges from a plaque (atherosclerotic buildup) within a large artery, such as the carotid artery or a significant branch of the circle of Willis. This clot then travels to a smaller artery in the brain, causing a temporary blockage. Emboli can also originate from the heart, especially in individuals with atrial fibrillation (an irregular heart rhythm) or heart valve abnormalities, where blood stasis or turbulence promotes clot formation.
Thrombotic Mini Strokes:
Thrombotic mini strokes result from forming a blood clot (thrombus) within a cerebral artery, usually at the site of an atherosclerotic plaque. The thrombus may wholly or partially occlude the artery, leading to transient ischemia in the affected brain region. Thrombotic mini-strokes often occur in individuals with underlying conditions such as atherosclerosis, hypertension, diabetes, or hyperlipidemia. These conditions increase the risk of plaque formation and thrombus development within cerebral arteries.
Hemodynamic Factors:
Mini-strokes can also result from transient decreases in cerebral blood flow beyond embolic and thrombotic mechanisms. Hemodynamic factors, such as hypotension, hypoperfusion, or vasospasm, can trigger these events. Individuals with impaired cerebral autoregulation, like those experiencing severe hypotension, cardiac arrhythmias, or carotid artery stenosis, are particularly vulnerable. In these cases, reduced blood flow leads to transient ischemia in susceptible brain regions.
Reperfusion and Resolution:
Unlike a full-blown stroke, which leads to permanent neurological deficits due to prolonged ischemic injury and infarction, mini-strokes present differently. The symptoms of mini-strokes are temporary and typically resolve quickly without causing lasting damage.
The temporary nature of mini-stroke symptoms is often due to the rapid restoration of blood flow, also known as reperfusion. This can occur through spontaneous clot lysis, the development of collateral circulation, or dynamic changes in vascular tone.
Although mini-stroke symptoms may resolve quickly, individuals must seek prompt medical evaluation. This helps identify and address underlying risk factors that could predispose them to future strokes.
In summary, a mini-stroke involves transient ischemia in the brain due to a temporary interruption of blood flow. This interruption is typically caused by embolic or thrombotic mechanisms or hemodynamic factors. Although mini-strokes share similar underlying mechanisms with full-blown strokes, their temporary symptoms distinguish them from permanent neurological damage. It is crucial to seek prompt evaluation and manage underlying risk factors to prevent recurrent strokes and optimize long-term outcomes for individuals who have experienced a mini-stroke.
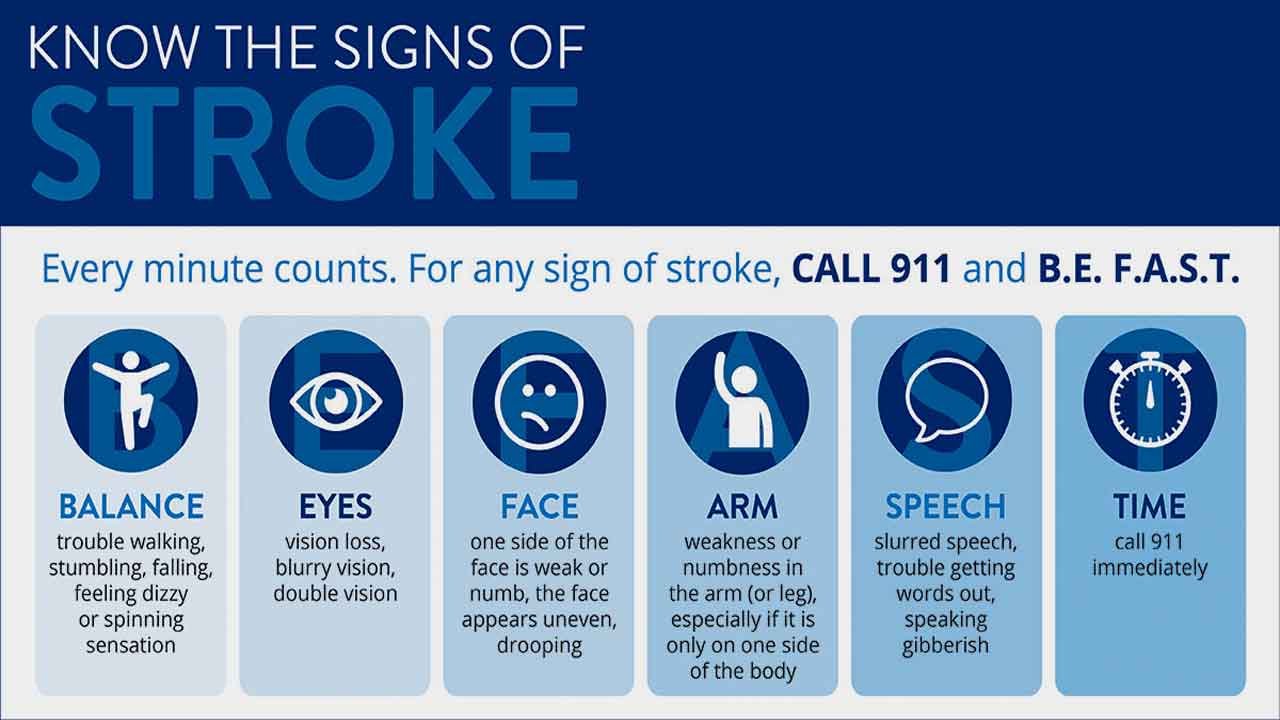
Symptoms of a Mini Stroke
Mini strokes, or transient ischemic attacks (TIAs), can present with various symptoms, each indicating a temporary disruption of blood flow to the brain. It’s important to note that not all symptoms may co-occur, and the severity can vary among individuals. Here’s a detailed list of common symptoms associated with mini-strokes:
Sudden Weakness or Numbness:
One of the hallmark symptoms of a mini-stroke is a sudden onset of weakness or numbness, often affecting one side of the body. This weakness or numbness can occur in the face, arm, or leg. It usually appears on the side of the body opposite to the affected brain hemisphere.
Difficulty Speaking or Understanding Speech (Dysphasia):
Another common symptom of a mini-stroke is difficulty speaking or understanding speech. Individuals may experience slurred speech, difficulty finding the right words (word-finding difficulty), or problems understanding spoken or written language.

Temporary Loss of Vision:
Mini strokes can cause temporary vision loss, often described as a curtain falling over one eye or a sudden blackout. Depending on the location and extent of the disruption in blood flow to the brain’s visual processing areas, vision loss may affect one eye or both eyes.
Dizziness and Loss of Balance:
Some individuals may experience dizziness or a sensation of spinning (vertigo) during a mini-stroke. Loss of balance or coordination may also occur, making it difficult to walk or maintain steady movement.
Brief Episodes of Confusion or Memory Loss:
Mini strokes can lead to temporary confusion, disorientation, or memory loss. Individuals may have difficulty concentrating, following conversations, or recalling recent events.
Trouble with Coordination:
Coordination difficulties, such as fine motor skills or clumsiness, may occur during a mini-stroke. This can manifest as difficulty performing tasks that require precise movements, such as writing or buttoning a shirt.
Facial Drooping:
In some cases, mini-strokes may cause facial drooping, similar to what is observed in full strokes. One side of the face may appear droopy or asymmetrical due to weakness or paralysis of the facial muscles.
Recognizing that these symptoms can vary in severity and duration is important. While they typically resolve within minutes to hours without causing permanent damage, they serve as warning signs of an increased risk of future strokes. Therefore, prompt medical attention is crucial if you or someone else experiences symptoms suggestive of a mini-stroke.
Duration and Residual Effects
Mini strokes typically last for a few minutes to hours, with symptoms resolving spontaneously. Unlike a full stroke, mini-strokes do not cause permanent brain damage or long-term disability. However, they serve as warning signs for an increased risk of future strokes, making prompt medical attention essential.
Understanding the duration and residual effects of mini-strokes, also known as transient ischemic attacks (TIAs), is essential for recognizing their temporary nature and potential impact on individuals’ health. Here’s a detailed description:
Duration of Symptoms:
- Mini strokes typically produce symptoms that come on suddenly and last for a relatively short duration, usually ranging from a few minutes to up to 24 hours.
- Most TIAs resolve spontaneously within minutes to hours, with symptoms gradually improving or disappearing altogether.
- In some cases, symptoms may persist for several hours but rarely last longer than 24 hours.
Transient Nature:
- The term “transient” in transient ischemic attack reflects the temporary nature of the symptoms.
- Unlike a full stroke, which results in permanent brain damage, the symptoms of a TIA resolve entirely, and there is no lasting impairment of brain function.
- Despite their transient nature, TIAs serve as warning signs of an increased risk of future strokes, making prompt medical evaluation and intervention crucial.
Residual Effects:
- In general, mini-strokes do not leave any residual effects or permanent damage to the brain.
- Once blood flow is restored to the affected area of the brain, brain function returns to normal, and individuals typically recover fully without lasting deficits.
- Unlike full strokes, which can cause paralysis, speech difficulties, cognitive impairment, or other long-term disabilities, TIAs do not result in lasting neurological deficits.
Warning Sign for Future Strokes:
- Although the symptoms of a TIA resolve spontaneously, they should not be ignored or dismissed.
- TIAs serve as warning signs that there is an underlying vascular problem or risk factor that needs to be addressed to prevent future strokes.
- Individuals who experience a TIA are at a significantly higher risk of experiencing a full stroke in the future, particularly within the days, weeks, or months following the TIA.
Importance of Medical Evaluation:
- It is crucial for individuals who experience symptoms of a mini-stroke to seek prompt medical evaluation.
- A thorough assessment by a healthcare professional can help determine the underlying cause of the TIA, identify any modifiable risk factors, and implement preventive measures to reduce the risk of future strokes.
- Diagnostic tests such as brain imaging (CT scan or MRI), carotid ultrasound, and electrocardiogram (ECG) may be performed to evaluate the extent of the vascular damage and assess the risk of future stroke.
In summary, mini-strokes are characterized by transient symptoms. These symptoms typically resolve within minutes to hours. Importantly, mini-strokes do not leave permanent damage.
However, despite their temporary nature, TIAs serve as warning signs. They indicate an increased risk of future strokes. Therefore, it is crucial to seek prompt medical evaluation. Additionally, modifying risk factors and implementing preventive measures are essential. These steps help reduce the likelihood of recurring vascular events.
The Role of Biofeedback in Mini Stroke Recovery at Home: Insights from Research Data
Mini strokes, also known as transient ischemic attacks (TIAs), serve as significant warning signs of potential future strokes. While prompt medical intervention and lifestyle modifications are crucial, rehabilitation strategies are vital in aiding mini-stroke recovery. In recent years, biofeedback has emerged as a promising therapeutic approach. This technique provides personalized, real-time feedback. Biofeedback aims to enhance motor and cognitive functions. Additionally, it helps improve functional abilities and promotes neuroplasticity.
Numerous studies have investigated the efficacy of biofeedback in stroke and TIA mini stroke recovery, demonstrating its potential to improve motor function, reduce disability, and enhance quality of life. Research data have shown that biofeedback interventions targeting upper limb function, balance, gait, and cognitive skills can yield positive outcomes in stroke survivors.
Although research on biofeedback for TIA mini stroke recovery is limited, we can apply principles from stroke rehabilitation studies. The transient nature of TIAs and the lack of long-term neurological deficits suggest that biofeedback could be beneficial. Tailoring biofeedback interventions to address specific impairments from mini-strokes might facilitate faster recovery. Additionally, these interventions could help reduce the risk of recurrent events.
Potential Benefits of Biofeedback in Mini Stroke Recovery
Research data suggest several potential benefits of integrating biofeedback into mini stroke rehabilitation programs:
- Enhancing Motor Recovery: Biofeedback techniques can promote motor learning and retraining, facilitating recovery of motor function in individuals affected by mini-strokes.
- Improving Cognitive Function: Cognitive rehabilitation using biofeedback may help address cognitive deficits commonly associated with TIAs, such as attention, memory, and executive functions.
- Promoting Neuroplasticity: Biofeedback-induced neurofeedback mechanisms may promote neuroplasticity changes in the brain, facilitating recovery and adaptive reorganization of neural networks following mini-strokes.
- Encouraging Active Participation: Biofeedback’s interactive nature allows individuals to actively engage in their rehabilitation process, fostering motivation, self-efficacy, and adherence to therapy.
Biofeedback Modalities in mini stroke recovery
By tailoring biofeedback modalities to address specific symptoms and deficits observed in individuals who have experienced a mini stroke, rehabilitation professionals can offer personalized and targeted interventions. These interventions aim to optimize recovery and improve functional outcomes. Additionally, selecting the appropriate biofeedback techniques should depend on the individual’s needs, goals, and clinical presentations. Choosing these techniques is crucial to carefully considering the underlying impairments and rehabilitation objectives.
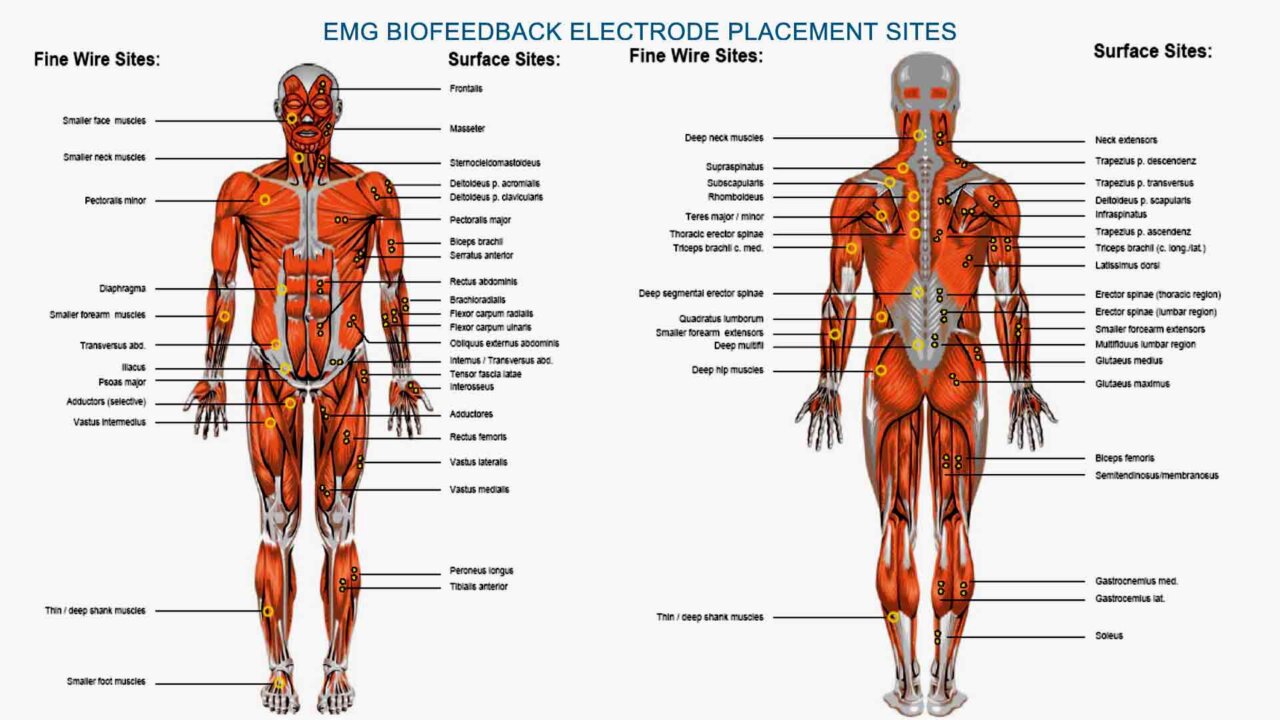
EMG Biofeedback in TIA mini stroke recovery at home
Using electromyography (EMG) biofeedback in TIA mini stroke recovery can target specific muscle groups affected by weakness or paralysis, helping individuals regain motor control and functional abilities. Here’s a detailed exploration of EMG biofeedback in mini stroke rehabilitation. First, we’ll discuss which muscles can be trained with this technique. Next, we’ll look at the intensity of the training required. Additionally, we’ll examine the potential benefits of combining EMG biofeedback with electrostimulation. Finally, we’ll review research data on the effectiveness of EMG biofeedback in stroke rehabilitation.
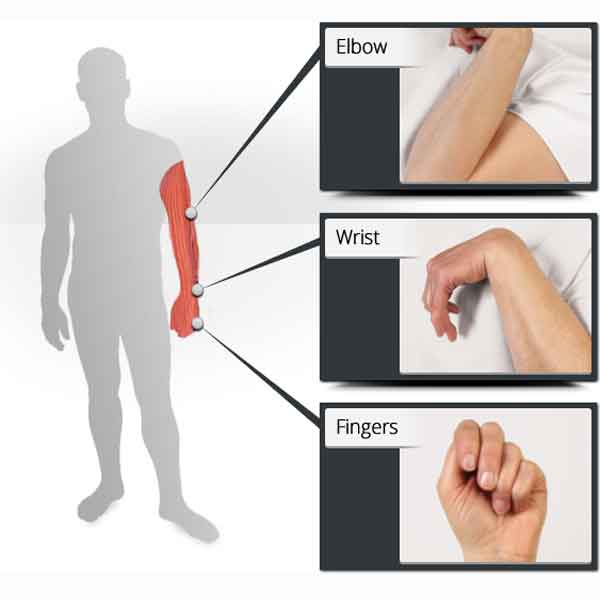
1. Muscles Targeted:
EMG biofeedback can train various muscle groups, depending on the individual’s impairments and rehabilitation goals.
Commonly targeted muscle groups in TIA mini stroke recovery at home include those involved in
- upper limb function (e.g., deltoids, biceps, triceps, wrist extensors/flexors),
- lower limb function (e.g., quadriceps, hamstrings, calf muscles),
- and trunk stability (e.g., abdominals, paraspinal muscles).
2. Intensity of Training:
The intensity of EMG biofeedback training can be adjusted based on the individual’s level of motor impairment, functional goals, and tolerance for physical activity.
- Training sessions typically involve repetitive exercises focused on activating and strengthening the targeted muscle groups.
- EMG biofeedback offers real-time feedback. This helps individuals learn to engage the correct muscles effectively. As a result, it improves their movement patterns. By using EMG biofeedback, individuals can enhance motor learning and neuromuscular reeducation.
3. Combining Biofeedback with Electrostimulation:
Combining EMG biofeedback with electrostimulation, such as functional electrical stimulation (FES) or neuromuscular electrical stimulation (NMES), may offer synergistic benefits in mini stroke rehabilitation.
- Electrostimulation delivers electrical impulses directly to the affected muscles. These impulses help activate the muscles, enhance muscle strength, and promote motor recovery.
- When used alongside EMG biofeedback, electrostimulation complements the feedback from EMG signals. It helps optimize muscle recruitment and promotes more efficient movement patterns.
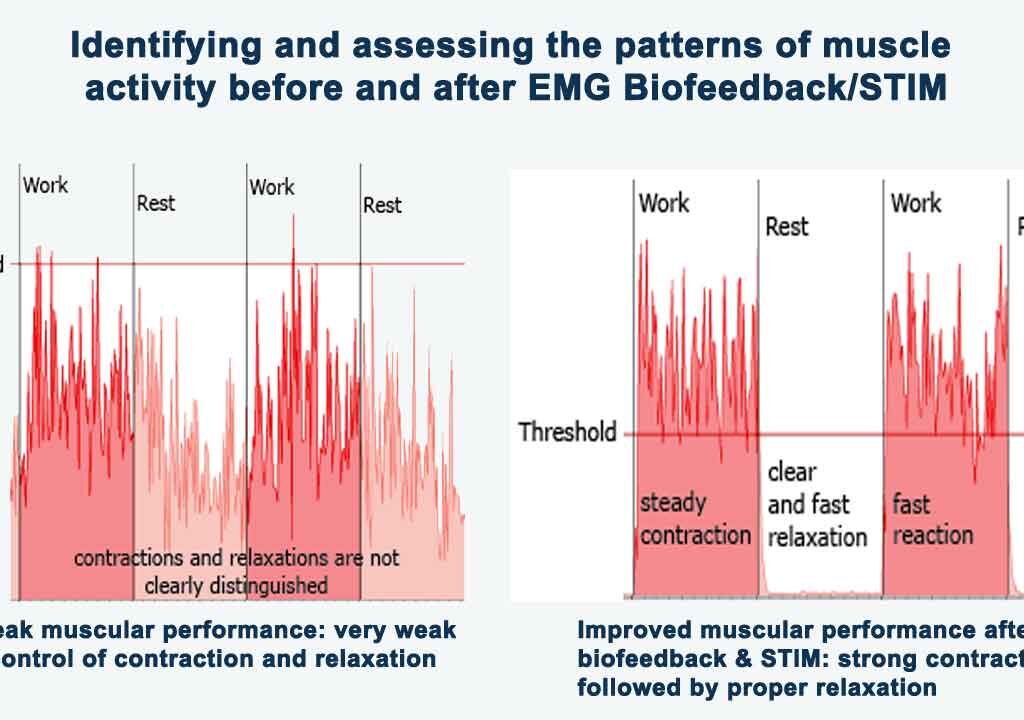
4. Research Data on Effectiveness:
Several studies have investigated the effectiveness of EMG biofeedback in stroke rehabilitation, including TIA mini stroke recovery at home, with promising results.
EMG biofeedback is a valuable modality in mini stroke rehabilitation. It enables individuals to target specific muscle groups, adjust training intensity, and optimize movement patterns through real-time feedback.
Combining EMG biofeedback with electrostimulation may offer additional benefits in promoting motor recovery and functional independence. Research data support the effectiveness of EMG biofeedback interventions in stroke rehabilitation, suggesting its potential utility in mini stroke recovery and prevention.
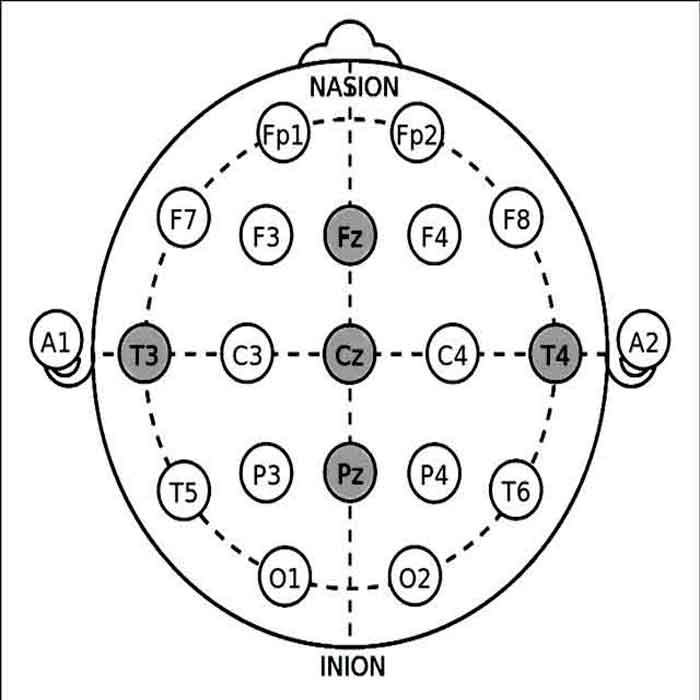
EEG Biofeedback (Neurofeedback) in TIA mini stroke recovery
Using electroencephalography (EEG) biofeedback, also known as neurofeedback, in TIA mini stroke recovery can target cognitive impairments, attention deficits, and other neurological symptoms by promoting neuroplasticity and enhancing brain function. Here’s a detailed exploration of EEG biofeedback in mini stroke rehabilitation, including neurofeedback protocols and application sites for different cases:
1. Neurofeedback Protocols:
Neurofeedback protocols involve training individuals to modulate their brainwave activity. They typically focus on specific EEG frequencies associated with cognitive functions and emotional regulation.
Standard neurofeedback protocols used in TIA mini stroke recovery include:
- Sensorimotor Rhythm (SMR) Training: SMR neurofeedback aims to enhance sensorimotor integration and attentional control by training individuals to increase SMR (12-15 Hz) activity over sensorimotor cortex areas.
- Theta/Beta Ratio Training: This protocol targets attention deficits and hyperarousal by teaching individuals to decrease theta (4-8 Hz) activity and increase beta (15-30 Hz) activity, particularly over frontal cortical regions.
- Alpha-Theta Training: Alpha-theta neurofeedback promotes relaxation, stress reduction, and emotional processing by guiding individuals to increase alpha (8-12 Hz) activity and induce theta (4-8 Hz) activity, typically over posterior cortical areas.
- Connectivity-Based Neurofeedback: This advanced protocol focuses on enhancing functional connectivity between brain regions associated with cognitive functions, such as attention, memory, and executive control.
2. Electrode Application Sites:
The selection of neurofeedback application sites depends on the specific cognitive deficits and neurological symptoms observed in individuals following a mini-stroke.
- For motor-related deficits (e.g., hemiparesis, impaired coordination), SMR training can target sensorimotor cortex areas contralateral to the affected limbs.
- Attention deficits and executive dysfunction may benefit from theta/beta ratio training or alpha-theta training, with electrodes placed over the frontal and prefrontal cortical regions.
- Emotional dysregulation, anxiety, or depression may be addressed through alpha-theta training or connectivity-based neurofeedback, targeting limbic system structures such as the amygdala and anterior cingulate cortex.
- Individualized neurofeedback protocols may involve a combination of training sites based on comprehensive assessment data, treatment goals, and patient-specific needs.
3. Integration with Cognitive Rehabilitation:
- Neurofeedback can be integrated into comprehensive cognitive rehabilitation programs for mini stroke recovery, complementing other therapeutic interventions such as mental training, psychoeducation, and cognitive behavioral therapy.
- Cognitive rehabilitation goals may include improving attention, memory, executive function, emotional regulation, and adaptive coping skills.
- Neurofeedback sessions can be tailored to reinforce cognitive skills and promote adaptive neural network changes, enhancing the efficacy of cognitive rehabilitation interventions.
Research Evidence and Effectiveness:
Research on the effectiveness of EEG biofeedback in mini stroke recovery is evolving, with promising findings suggesting its potential benefits in enhancing cognitive function and neurological outcomes.
EEG biofeedback offers a promising approach in mini stroke rehabilitation. Through personalized neurofeedback protocols, it targets cognitive impairments, attention deficits, and emotional dysregulation. By promoting neuroplastic changes in brain function and connectivity, EEG biofeedback contributes to optimizing cognitive rehabilitation outcomes and enhancing neurological recovery following a mini-stroke.
Non-EEG Near-Infrared Spectroscopy (NIRS) Neurofeedback in recovery
The Mendi Headband employs near-infrared spectroscopy (NIRS) to measure changes in brain activity. This optical technology monitors blood flow and oxygenation levels in the prefrontal cortex, providing insights into brain function without needing electrodes or complex setups.
The Mendi Headband can support rehabilitation after a stroke by focusing on cognitive functions and neural plasticity. Here’s how it works:
Enhancing Neural Plasticity:
The brain changes neural connectivity and function after a stroke. The Mendi Headband utilizes neurofeedback through near-infrared spectroscopy (NIRS) to provide real-time feedback on brain activity in the prefrontal cortex. Engaging in brain training exercises with the headband stimulates neural plasticity. Neural plasticity is the brain’s ability to reorganize and form new connections. This process is crucial for recovering lost cognitive functions.
Improving Cognitive Functions:
Stroke survivors often experience cognitive impairments such as attention, memory, and decision-making difficulties.
The Mendi Headband helps users focus on enhancing these cognitive functions by providing feedback that encourages activating specific brain areas involved in these processes. Over time, this targeted brain training can aid in the recovery of mental abilities that may have been affected by the stroke.
Facilitating Rehabilitation Exercises:
The headband can complement traditional therapies during rehabilitation sessions. Engaging in cognitive tasks and receiving immediate feedback on brain activity can benefit stroke patients. This approach helps them optimize their rehabilitation efforts. As a result, it may potentially accelerate their recovery process.
Supporting Mental Well-being:
Rehabilitation after a stroke can be emotionally challenging. The Mendi Headband provides more than just cognitive enhancement. It also promotes relaxation and reduces stress levels. These benefits contribute to improved overall mental well-being during recovery.
Long-term Benefits:
Continued use of the Mendi Headband in stroke rehabilitation may improve cognitive functions and overall brain health. It serves as a tool for ongoing cognitive maintenance and enhancement, helping individuals regain independence and quality of life after a stroke.
In summary, the Mendi Headband supports stroke rehabilitation by promoting neural plasticity, enhancing cognitive functions, facilitating rehabilitation exercises, supporting mental well-being, and offering long-term cognitive benefits.
Breathing Biofeedback in mini stroke recovery
Respiratory, or breathing, biofeedback is another modality that can be utilized in mini stroke rehabilitation, particularly for addressing symptoms related to stress, anxiety, and respiratory dysfunction. Here’s how respiratory biofeedback can be beneficial in managing certain aspects of mini stroke recovery.
Stress and Anxiety Reduction:
- Many individuals who have had a mini-stroke may experience increased stress and anxiety. This can result from the event itself or worries about future health risks.
- Respiratory biofeedback can assist individuals in regulating their breathing patterns. It teaches techniques such as diaphragmatic breathing, paced breathing, and coherent breathing. These methods help induce relaxation.
- Respiratory biofeedback monitors parameters like respiratory rate, depth of breathing, and heart rate variability. It provides real-time feedback to guide individuals. This helps them achieve a calm and balanced breathing rhythm. As a result, it reduces stress and anxiety levels.
Management of Respiratory Dysfunction:
Mini strokes can occasionally affect brain regions involved in respiratory control, leading to respiratory dysfunction or irregular breathing patterns.
- Respiratory biofeedback techniques can assist individuals in improving respiratory function by promoting optimal breathing patterns and lung capacity.
- Through visual or auditory feedback, individuals can learn to adjust their breathing rate, depth, and rhythm to optimize oxygenation, reduce respiratory effort, and enhance overall respiratory efficiency.
Promotion of Relaxation and Well-being:
Respiratory biofeedback fosters mindfulness and body awareness, encouraging individuals to focus on their breath and engage in relaxation practices.
- By incorporating mindfulness meditation or relaxation training elements, respiratory biofeedback sessions can help individuals cultivate a sense of inner calm, reduce muscle tension, and enhance overall well-being.
- Regular practice of respiratory biofeedback techniques can empower individuals to manage stressors better, improve emotional resilience, and promote a sense of control over their physiological responses.
Complementary Therapy for Comprehensive Rehabilitation:
Respiratory biofeedback can complement other rehabilitation interventions, such as physical therapy, occupational therapy, and cognitive-behavioral therapy, in a comprehensive mini stroke rehabilitation program.
- Integrating respiratory biofeedback into multidisciplinary treatment plans provides individuals with additional tools for managing their physical, emotional, and cognitive recovery journey.
- By addressing the physiological and psychological dimensions of mini stroke recovery at home, respiratory biofeedback contributes to a holistic approach to rehabilitation, promoting overall health and resilience.
Respiratory biofeedback is a valuable modality in mini stroke rehabilitation. It offers benefits such as stress reduction, respiratory dysfunction management, relaxation promotion, and overall well-being enhancement. Respiratory biofeedback teaches individuals to regulate their breathing patterns and cultivate a sense of inner calm. This empowerment allows them to actively participate in their recovery process. As a result, it can significantly improve their quality of life following a mini-stroke.
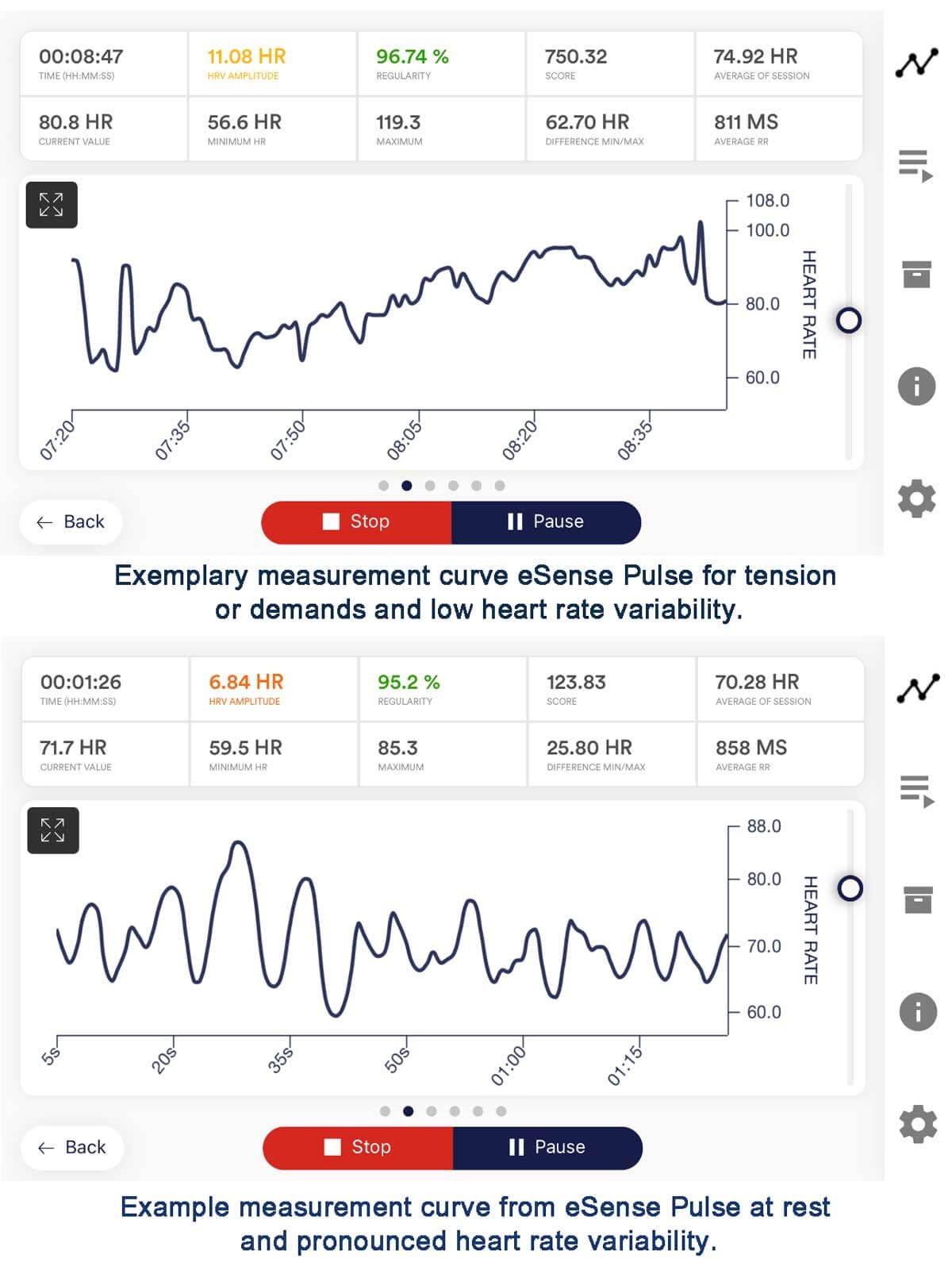
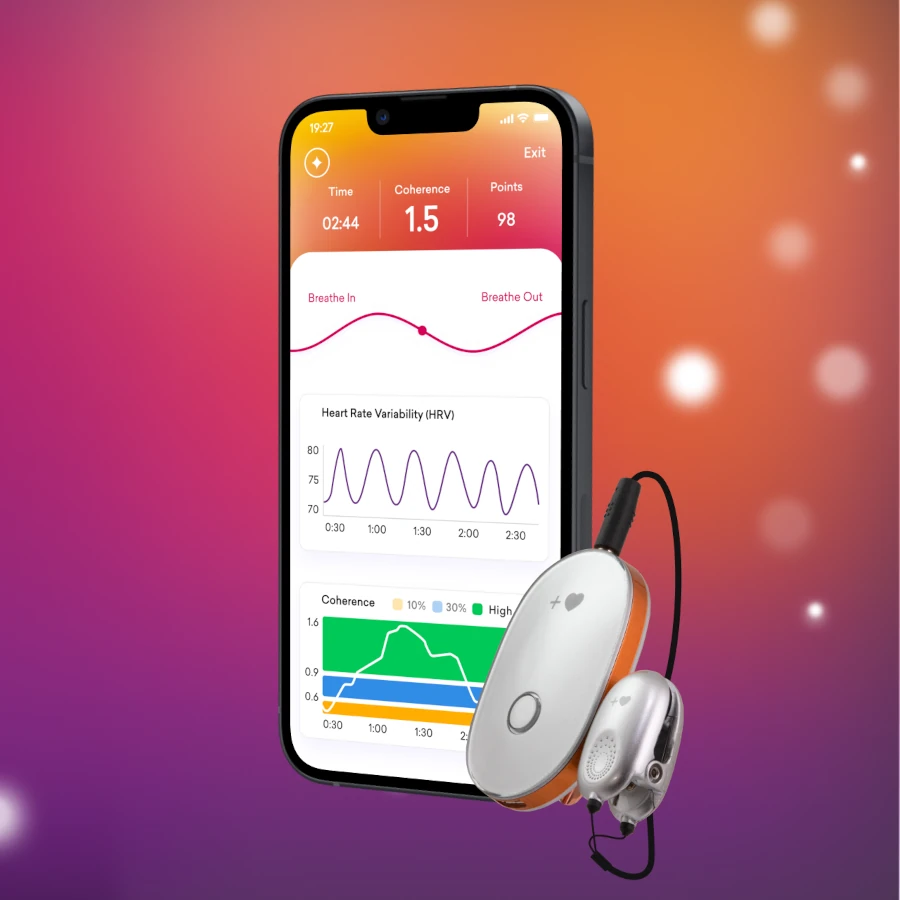
Heart Rate Variability Biofeedback in mini stroke recovery
Heart rate variability (HRV) biofeedback is a noninvasive technique that provides real-time feedback to train individuals to regulate their HRV, which reflects the balance between sympathetic and parasympathetic activity of the autonomic nervous system. In the context of TIA mini stroke recovery, HRV biofeedback offers a promising approach. Specifically, it enhances physiological resilience, reduces stress, alleviates anxiety, addresses emotional dysregulation, and promotes overall well-being.
Principles of Heart Rate Variability Biofeedback
HRV biofeedback is based on the concept that more significant variability in the timing between heartbeats (inter-beat intervals) reflects a healthier autonomic nervous system function and greater adaptability to stressors.
Fundamental principles of HRV biofeedback include:
1. Real-Time Feedback: Individuals receive visual or auditory feedback on their heart rate variability, typically in the form of a computer-generated display or sound. This allows them to observe changes in their physiological state and adjust their breathing and mental focus accordingly.
2. Resonant Frequency Breathing: HRV biofeedback often incorporates resonant frequency breathing techniques, which involve breathing at a specific rate (usually around six breaths per minute) to maximize heart rate variability and promote relaxation.
3. Self-Regulation: Through practice and repetition, individuals learn to modulate their heart rate variability through conscious control of their breathing patterns, mental focus, and emotional state, enhancing their ability to self-regulate physiological responses to stressors.
Application of HRV Biofeedback in Mini Stroke Recovery:
In the context of mini-stroke recovery, HRV biofeedback can address several aspects of rehabilitation and promote overall recovery and well-being:
Stress Reduction and Emotional Regulation:
Mini strokes and their aftermath can be emotionally challenging, leading to increased stress and anxiety. HRV biofeedback teaches individuals to induce a state of physiological relaxation and emotional calmness, leading to reduced sympathetic arousal and increased parasympathetic activity, reducing the negative impact of stress on cardiovascular health and promoting faster recovery.
Autonomic Balance:
Imbalances in autonomic nervous system function, such as increased sympathetic activity and decreased parasympathetic activity, are common in individuals with a history of stroke. HRV biofeedback helps restore autonomic balance by strengthening parasympathetic tone. It also reduces sympathetic arousal. As a result, cardiovascular function improves, and the risk of recurrent strokes decreases.
Neuroplasticity and Cognitive Rehabilitation:
HRV biofeedback may promote neuroplasticity changes in the brain by modulating autonomic nervous system activity and promoting optimal cerebral perfusion. These neuroplasticity effects can support recovery of cognitive function, memory, attention, motor skills, emotional resilience, and executive function following a mini-stroke. By promoting neuroplasticity, HRV biofeedback may enhance the brain’s ability to adapt and reorganize in response to injury, facilitating functional recovery and improving overall cognitive outcomes.
Secondary Stroke Prevention:
By teaching individuals to self-regulate their physiological responses and reduce modifiable risk factors such as stress, hypertension, and inflammation, HRV biofeedback can contribute to secondary stroke prevention and long-term vascular health.
Regular practice of HRV biofeedback techniques can lead to sustained improvements in autonomic function and blood pressure control. Consequently, this reduces the likelihood of recurrent strokes. Additionally, it enhances overall cardiovascular health and improves the prognosis.
Integration with Comprehensive Rehabilitation Programs:
HRV biofeedback should be integrated into comprehensive stroke rehabilitation programs, complementing other therapeutic interventions such as physical therapy, occupational therapy, speech therapy, and cognitive rehabilitation. By addressing both physiological and psychological aspects of recovery, HRV biofeedback enhances the effectiveness of multidisciplinary rehabilitation efforts and promotes holistic recovery from mini-strokes.
Heart rate variability biofeedback offers a promising adjunctive approach to mini stroke recovery by promoting stress reduction, autonomic balance, neuroplasticity, and secondary stroke prevention. Further research is warranted to elucidate the specific effects of HRV biofeedback on stroke outcomes and optimize its integration into comprehensive rehabilitation programs for individuals with a history of mini-strokes.
Temperature and ESR (Electrodermal Activity and Skin Resistance) biofeedback in mini stroke recover
Temperature and ESR (Electrodermal Activity and Skin Resistance) biofeedback are less commonly utilized in mini stroke recovery compared to other modalities such as EMG (Electromyography) or EEG (Electroencephalography) biofeedback. However, they may still have potential applications in certain aspects of rehabilitation. Here’s how temperature and ESR biofeedback could theoretically be used in mini stroke recovery:
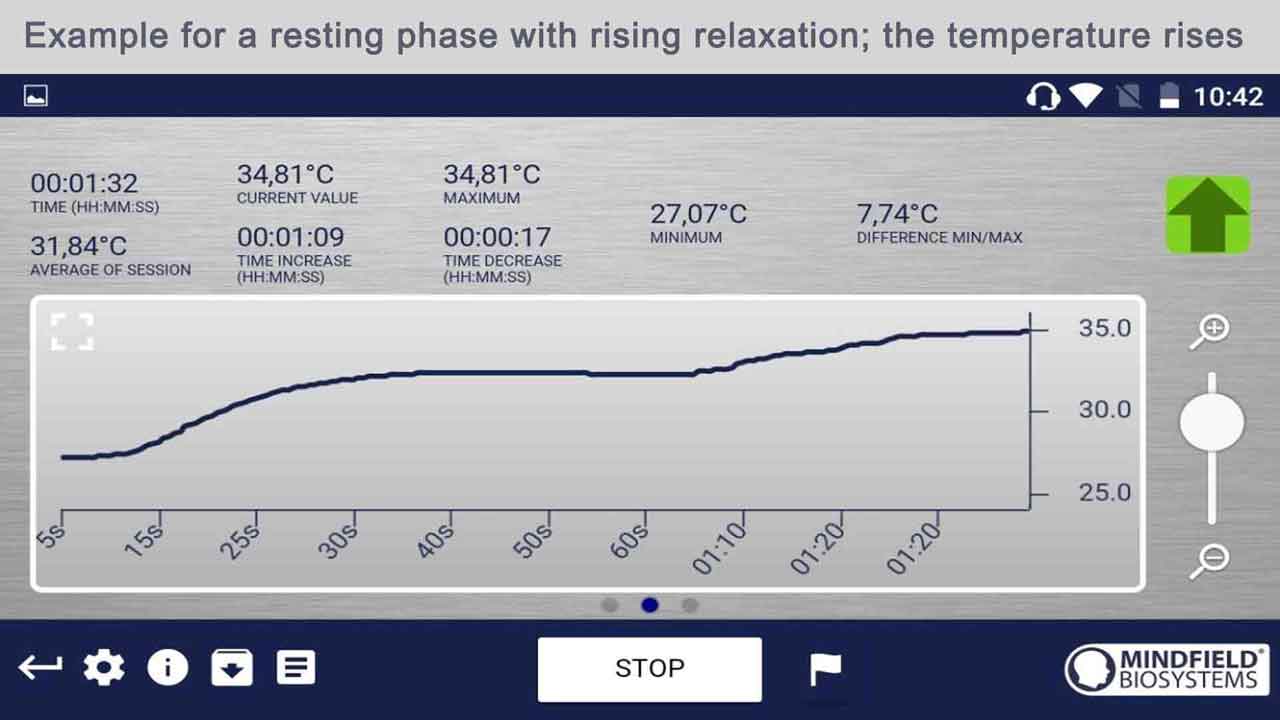
Temperature Biofeedback
- Temperature biofeedback involves monitoring and providing feedback on skin temperature, typically through sensors attached to the fingers or other peripheral areas.
- While there is limited research on temperature biofeedback in stroke rehabilitation, it has been used in other contexts, such as stress management and relaxation training.
- In mini stroke recovery, temperature biofeedback could promote relaxation, reduce stress, and enhance peripheral circulation, which may have secondary benefits for overall well-being and recovery.
- Individuals recovering from a mini stroke may experience heightened stress or anxiety, and temperature biofeedback could provide a non-invasive, self-regulatory technique for managing these emotional responses.
ESR Biofeedback (Electrodermal Activity and Skin Resistance)
- ESR biofeedback involves monitoring skin conductance or resistance changes, which reflect sympathetic nervous system activity and emotional arousal.
- Like temperature biofeedback, ESR biofeedback has been primarily used in stress management and anxiety reduction interventions.
- In mini stroke recovery, ESR biofeedback could help individuals regulate their autonomic nervous system responses, reduce emotional arousal, and promote relaxation.
- By modulating skin conductance or resistance levels through biofeedback training, individuals may develop greater awareness and control over their physiological stress responses, which could contribute to overall well-being and recovery.
While temperature and ESR biofeedback have theoretical potential in mini stroke recovery, it’s important to note that their effectiveness and specific applications in this context have not been extensively studied. As such, they are not typically considered primary interventions in stroke rehabilitation protocols. However, they may be used as adjunctive or complementary techniques in comprehensive rehabilitation programs, particularly for addressing emotional and psychophysiological factors that can impact recovery outcomes.
Before implementing temperature or ESR biofeedback in mini stroke rehabilitation, healthcare professionals must conduct a thorough assessment, consider individualized treatment goals, and ensure that the chosen interventions align with the patient’s needs and preferences. Additionally, further research is needed to evaluate the efficacy and potential benefits of these biofeedback modalities in mini stroke recovery.
The role of Biofeedback modalities in mini stroke prophylaxis
Biofeedback techniques have the potential to play a role in the prophylaxis or prevention of recurrent strokes after mini stroke recovery. While biofeedback is typically associated with rehabilitation and symptom management, it can also be utilized as a preventive measure to address underlying risk factors and promote healthy behaviors. Here’s how biofeedback may contribute to stroke prevention after mini stroke recovery:
Blood Pressure and Stress Management:
- Hypertension (high blood pressure) is a significant risk factor for stroke, including mini-strokes. Biofeedback techniques, such as heart rate variability (HRV) biofeedback and relaxation training, can help individuals regulate their autonomic nervous system responses, lower stress levels, and reduce blood pressure.
- By learning to modulate physiological markers of stress and arousal through biofeedback, individuals can adopt healthier coping strategies, manage hypertension, and reduce the risk of recurrent strokes.
Lifestyle Modification:
- Biofeedback interventions can support lifestyle modifications aimed at reducing stroke risk factors such as obesity, sedentary behavior, and unhealthy diet. For example, biofeedback can promote physical activity adherence, encourage mindful eating habits, and reinforce relaxation techniques to combat stress-related eating.
- Biofeedback empowers individuals to make positive changes and maintain healthier habits over the long term by providing real-time feedback on physiological responses to lifestyle behaviors, thus lowering their risk of future strokes.
Medication Adherence:
- Medication non-adherence is a common issue in stroke prevention, particularly among individuals with multiple comorbidities. Biofeedback can be integrated into medication adherence interventions by reinforcing positive behaviors and providing feedback on physiological markers associated with stress reduction and relaxation.
- Through biofeedback-enhanced interventions, individuals may develop greater motivation, self-efficacy, and accountability in managing their medications and following prescribed treatment regimens, thereby reducing the risk of recurrent strokes.
Cognitive and Emotional Health:
- Cognitive impairments and emotional distress are associated with an increased risk of stroke recurrence. Biofeedback techniques targeting cognitive function, attention, and emotional regulation can support ongoing cognitive rehabilitation efforts and promote resilience against future strokes.
- By incorporating cognitive and emotional health components into biofeedback-based interventions, individuals can develop adaptive coping strategies. Additionally, they can enhance cognitive resilience. This approach also helps mitigate the impact of psychological risk factors on stroke recurrence.
While biofeedback interventions have the potential to contribute to stroke prevention after mini stroke recovery, it’s essential to recognize that they are most effective when integrated into comprehensive secondary prevention strategies. These strategies should include medication management, lifestyle modifications, regular medical monitoring, and ongoing education and support for individuals and their caregivers. Additionally, further research is needed to evaluate biofeedback-based prophylaxis interventions’ long-term effectiveness and sustainability in reducing stroke recurrence rates and improving overall outcomes.
Future Directions and Considerations
While the potential benefits of biofeedback in mini stroke recovery are promising, further research is needed to establish its efficacy, optimal parameters, and long-term outcomes in this population. Large-scale clinical trials, standardized protocols, and comparative effectiveness studies are warranted to validate the role of biofeedback as an adjunctive therapy in mini stroke rehabilitation. Additionally, accessibility, cost-effectiveness, and patient preferences should be considered when integrating biofeedback into clinical practice.
Conclusion
Biofeedback holds promise as a valuable adjunctive therapy in mini stroke recovery, offering personalized and targeted interventions to enhance motor and cognitive functions. While research data supporting its efficacy in this population are limited, insights from stroke rehabilitation studies underscore its potential benefits. Further research is needed to elucidate the optimal use of biofeedback techniques in mini stroke rehabilitation and to translate these findings into clinical practice for improved outcomes and enhanced quality of life for individuals affected by TIAs.
FAQ: Mini Strokes and Biofeedback Therapy
The key difference lies in symptom duration and the presence of permanent damage. A mini-stroke, or Transient Ischemic Attack (TIA), causes temporary symptoms that typically resolve within 24 hours and do not result in permanent brain damage. A full stroke, however, involves a prolonged interruption of blood flow, leading to permanent neurological deficits.
Common symptoms of a mini-stroke are sudden and may include:
- Weakness or numbness in the face, arm, or leg, often on one side of the body.
- Difficulty speaking or understanding speech (dysphasia).
- Temporary vision loss in one or both eyes.
- Dizziness, loss of balance, or lack of coordination.
- Brief episodes of confusion or memory loss.
Yes, the symptoms of a mini-stroke are transient and typically resolve completely without permanent brain damage. However, a TIA is a critical warning sign of an increased risk of a future, more severe stroke, making immediate medical evaluation essential.
Biofeedback is a therapeutic approach that uses real-time electronic data to help patients gain control over physical processes. In mini-stroke recovery, it can:
- Enhance motor recovery by retraining specific muscle groups.
- Improve cognitive functions like attention and memory.
- Promote neuroplasticity, the brain’s ability to reorganize and form new connections.
- Reduce stress and anxiety, which are common after a TIA.
EMG (Electromyography) Biofeedback targets specific muscle groups affected by weakness or paralysis. It provides real-time feedback on muscle activity, helping individuals relearn how to activate and control these muscles, thereby improving motor function, strength, and coordination.
List of References
- Chouinard, P. A., & Paus, T. (2010). What have we learned from “Perturbing” the human cortical motor system with transcranial magnetic stimulation? Frontiers in Human Neuroscience, 4, 173. doi:10.3389/fnhum.2010.00173.
- Duncan, P. W., Zorowitz, R., et al. (2005). Management of adult stroke rehabilitation care: A clinical practice guideline. Stroke, 36(9), e100–e143. doi:10.1161/01.STR.0000180861.54180.FF
- Gruzelier, J. H. (2014). EEG-neurofeedback for optimizing performance. III: A review of methodological and theoretical considerations. Neuroscience & Biobehavioral Reviews, 44, 159–182. doi:10.1016/j.neubiorev.2013.09.015
- Kober, S. E., Witte, M., et al. (2013). Learning to modulate one’s brain activity: The effect of spontaneous mental strategies. Frontiers in Human Neuroscience, 7, 695. doi:10.3389/fnhum.2013.00695
- Norouzi-Gheidari, N., Archambault, P. S., et al. (2012). Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: Systematic review and meta-analysis of the literature. Journal of Rehabilitation Research and Development, 49(4), 479–496. doi:10.1682/JRRD.2010.10.0210
- Rehme, A. K., & Grefkes, C. (2013). Cerebral network disorders after stroke: Evidence from imaging-based connectivity analyses of active and resting brain states in humans. The Journal of Physiology, 591(1), 17–31. doi:10.1113/jphysiol.2012.243469
- Sitaram, R., Ros, T., et al. (2016). Closed-loop brain training: The science of neurofeedback. Nature Reviews Neuroscience, 18(2), 86–100. doi:10.1038/nrn.2016.164
- Yang, Q., Wu, S., et. (2020). The effects of biofeedback therapy on upper limb function after stroke: A meta-analysis of randomized controlled trials. Journal of Stroke and Cerebrovascular Diseases, 29(7), 104870. doi:10.1016/j.jstrokecerebrovasdis.2020.104870